When it comes to irrigation water and water quality, you’ve probably heard the terms pH, alkalinity and bicarbonates thrown around. In this article, we’ll break down these terms and explain which should be the focus when treating your irrigation water.
pH (potential Hydrogen) – a logarithmic scale measuring the acidity [hydrogen cation (H+) concentration] and alkalinity [hydroxide anion (OH–) concentration] of a solution.
How Do I Treat My Water Source?
Many growers focus on pH targets when adjusting their irrigation water. This is likely based on the fact that there is an optimal pH range for growing media that allows for the solubility and uptake of all essential plant nutrients:
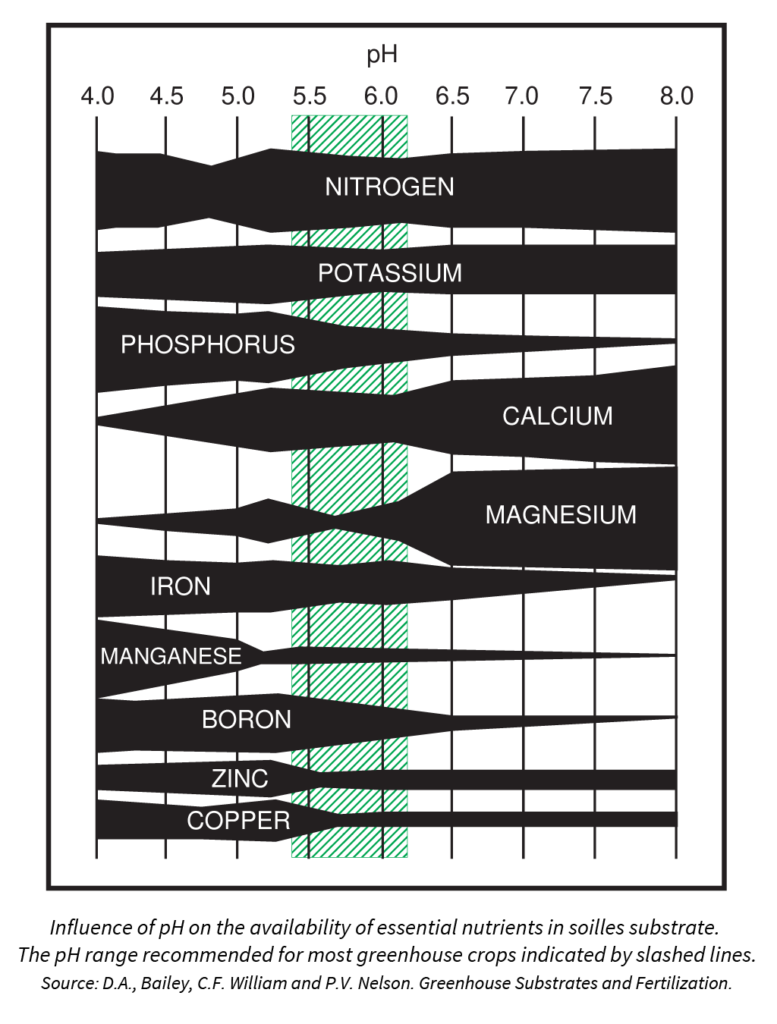
In reality, the pH of your irrigation water has very little impact on the pH of your growing media. (More about this below.)
The problem with targeting pH when adjusting irrigation water is that it’s a relative reading that doesn’t give us a clear picture of how the water will tolerate the addition of acid or base to the system. The Acid Neutralizing Capacity (ANC) or Alkalinity will dictate that.
Alkalinity – a measure of the capacity of water to resist changes in pH with the addiction of acid; the combined amount of carbonates (CO32-), bicarbonate (HCO3–) and hydroxide ions (OH–) in a solution.
These ions are all part of the carbonate/bicarbonate buffering system, something that our own bodies use to regulate blood pH.
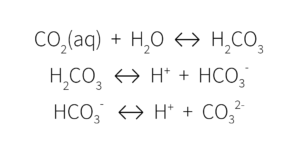 This multistep decomposition of carbonic acid interacts with plant nutrients in the growing media, ultimately impacting media pH. When alkalinity is high, these ions remove the H+ ions, lowering the acidity and increasing the pH.
This multistep decomposition of carbonic acid interacts with plant nutrients in the growing media, ultimately impacting media pH. When alkalinity is high, these ions remove the H+ ions, lowering the acidity and increasing the pH.
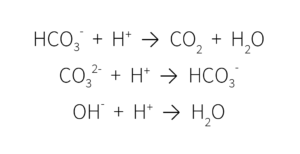 When the pH of growing media is too high, most micronutrients become unavailable and there is a risk of nutrient deficiencies.
When the pH of growing media is too high, most micronutrients become unavailable and there is a risk of nutrient deficiencies.
Bicarbonate – HCO3– – ion produced at the midpoint of carbonic acid decomposition.
When addressing water quality and the need for acid, we tend to focus on bicarbonate ions since this is the most prevalent ion in typical sources of fresh water.
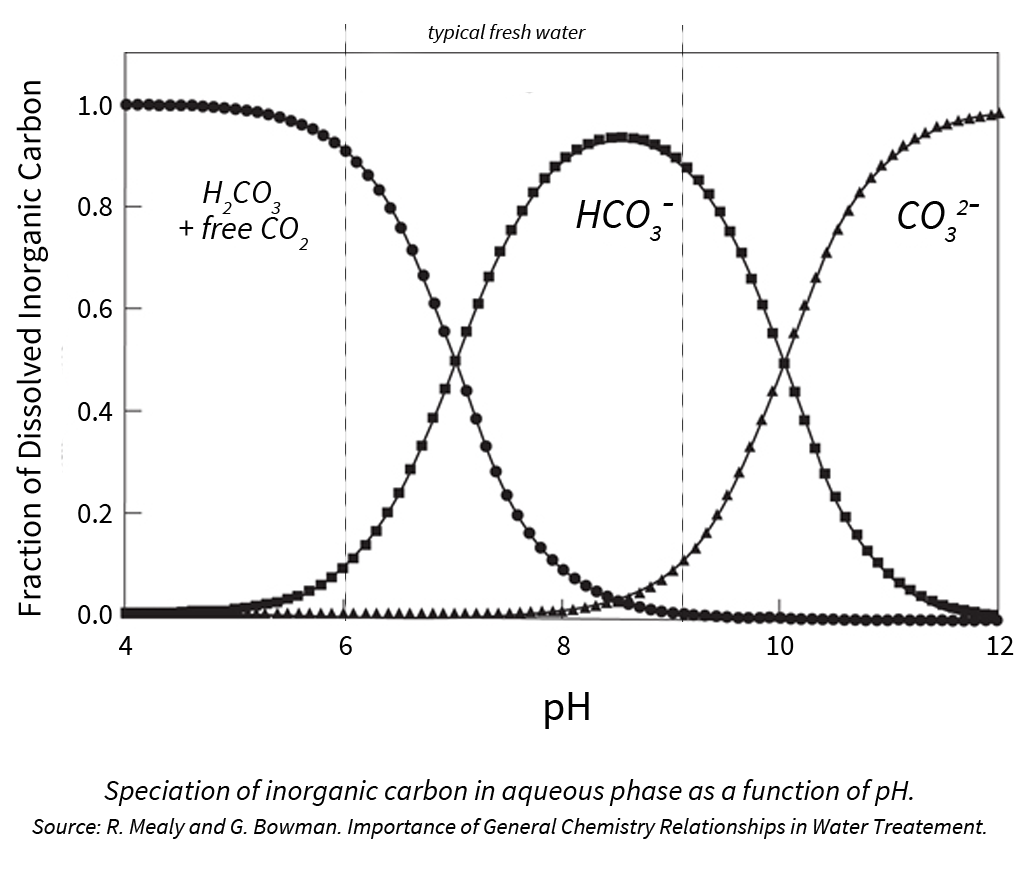
When bicarbonate levels are too high, they can react with calcium and magnesium to form bicarbonate salts, further increasing media pH and removing vital nutrients from solution.
When bicarbonate levels are too low, such as the case with Reverse Osmosis water, there is no buffering capacity, allowing inputs to have a strong impact on pH, typically resulting in significant swings in media pH. This can be remedied by adding potassium bicarbonate to your filtered water.
The ideal bicarbonate range is between 60-100 ppm, low end of range for younger plants. This level allows for an adequate buffering capacity while minimizing the influence on media pH.
The amount of acid required to neutralize bicarbonates will depend on the type and strength of acid that you’re using. Need help determining this? Feel free to get in touch – mppi@plantprod.com
